The IR Revolution
 INTERFEROGRAPHS
INTERFEROGRAPHS
Real world samples are often compromised in that they are lipemic, hemolyzed or icteric. Professors Glick and Ryder at Indiana University have called the attention of the clinical chemistry profession to serious errors caused by these compromised samples.
Depending on the patient population, 20-30% of the samples presented to hospital laboratories for testing are compromised with one or more of these interfering problems.
Even very low levels of these substances invalidate the results of methods in wide use.
This treatise will discuss the theory of infrared detection and its use in virtually eliminating these effects and then discuss practical effects on the results you will see.
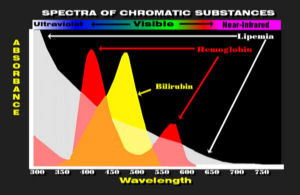 For decades, clinical chemistry tests have been made by causing a dye to form the concentration of which is directly proportional to the concentration of the analyte being measured.
For decades, clinical chemistry tests have been made by causing a dye to form the concentration of which is directly proportional to the concentration of the analyte being measured.
The dyes are then detected spectrophotometrically between 340 nm in the U.V. and 600 nm in the red region of the spectrum.
Hemoglobin, bilirubin and lipemia have substantial absorbance in this region of the spectrum and their absorbance can be mistaken for the dye being measured.
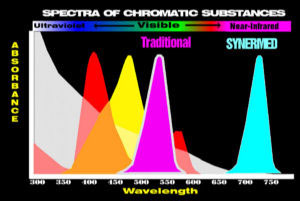 Most traditional methods develop colored substances (dyes) which are visible and are colored red or yellow. Many of the dyes in use today were first used between 1875 and 1920.
Most traditional methods develop colored substances (dyes) which are visible and are colored red or yellow. Many of the dyes in use today were first used between 1875 and 1920.
Other traditional methods measure coenzymes at 340 nm in the ultraviolet region.
Synermed has synthesized new colorless dyes (chromogens) which form invisible infrared absorbing dyes. Much of the process of converting chromogens to dyes using enzymes is unchanged. Only the detection of the dye in the infrared region is changed so that the specificity of the enzymes is retained and differences only appear with compromised samples.
The interfering absorbance of lipemia (milky serum) progressively decreases from the U.V. to the visible region of the spectrum.
LIPEMIA
Lipemia Lipemia is caused by fat droplets (chylomicra) suspended in water. Apparent absorbance decreases as wavelength increases due to refraction. When white light passes through a prism shorter wavelengths show the greatest refraction.
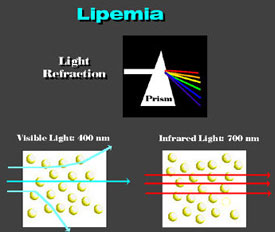
Thus, red then yellow, green, blue and then U.V. light appear as they are progressively bent by the prism. Infrared light passes through the prism without being bent. Small particles of fat have a higher refractive index than water and act as prisms, since the path through their periphery is shorter than at the center. Visible light is scattered by droplets while infrared light passes without its course being altered. Rainbow Fog and clouds are water droplets suspended in air. In this case, the water has a higher refractive index than the air. Correspondingly, white light is refracted by the water droplets in accordance with wavelength causing the well known rainbow to form.
During WWII, infrared photography was developed to take pictures of ground targets without interference from clouds or fog. Following the cold war, considerable infrared optical and detection capability has been made available to the private sector. (This means they now have something even better.) Synermed is the only company in the world applying these capabilities to clinical chemistry because only Synermed has developed the required infrared-dye producing chromogens.
SPECTROPHOTOMETRY
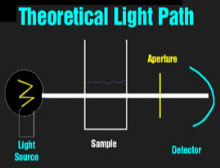 |
 |
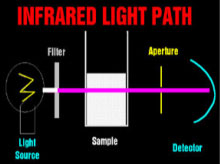 |
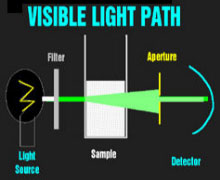
Theoretical Light PathVisible Light PathInfrared Light Path The spectrophotometers that are used universally to detect dyes in clinical chemistry are relatively simple devices. A light source (usually a halogen bulb) emits white light which is made monochromatic and then columnated to pass through the sample to a detector. One or more apertures (or fiberoptic bundles) screens uncolumnated light. When visible light passes through a milky reagent, the columnating optics are defeated and the apertures exclude light which is then undetected. This produces an apparent increase in absorbance. Infrared light is not scattered by milky samples and the spectrophotometer's optics perform as they were designed and no apparent absorbance of the sample is seen.
COMPARISON WITH REFERENCE LABORATORIES
Reference laboratories have not yet converted to the interference-free infrared methods. Thus, differences are likely to be seen when comparing results where the sample contains lipemia, hemolysis, blood substitutes or elevated levels of bilirubin.
Particularly troubling is the fact that commonly used reference laboratory methods may be affected by hemolysis at levels below that which can be visibly detected.
The Synermed total and direct bilirubin methods have been compared on thousands of samples by a prominent University center in Paris. They found depressed values in the reference method at levels below visible detection. The graph below compares the Synermed methods (Green) with the reference Jendrassik and Grof method (Red).
CALIBRATION
Multianalyte calibrators supplied by the manufacturers of automation are "fudged" to correct the calibration for the effects of chromatic substances in the calibrator. These calibrators only work with the instrument for which they are intended as the degree of chromatic interference varies from instrument to instrument.
Some manufacturers even give different calibrator values for different methods. Thus, enzymatic creatinine and Jaffé creatinine calibration values may vary by 20%.
The analytes which are adjusted the most are the low molar concentration analytes which are most affected by these substances.
Multianalyte calibrators are often used to calibrate total and direct bilirubin. Consequently, they contain high levels of these substances relative to normal serum. Although bilirubin interference is far from the most common chromatic interference in patient samples, it is a major cause of value adjustment in calibrators.
Optimal applications of Synermed's infrared methods exhibit virtually none of these effects and calibrators "fudged" to correct visible methods will produce false values.
Synermed produces IRCal calibrators for the calibration of the infrared methods. The product is traceable to NIST (National Bureau of Standards).
CORRELATION STUDIES
Two factors are necessary to achieve correlation of the Synermed® infrared methods with traditional methods:
Calibration rationalization to achieve the same mean; and
The use of non-chromatically compromised samples.
Chromatically compromised samples will not correlate; the fact that results are different is the point of using the Synermed® infrared methods.
The Synermed bilirubins and creatinine represent such an improvement that high correlation may be difficult.
SOME ANSWERED QUESTIONS
Are normal values different?
In theory, normal values should be no different for the infrared values since the principal difference is freedom from chromatic interferences. Since truly normal samples are not hemolyzed or lipemic or icteric, the values should be the same. However, some systems use very wide ranges to take into account the imprecision of the system.
Some of the Synermed® methods have greatly improved chemical specificity as discussed below. In these few cases, normal values must be re-examined or normalized to traditional values.
What differences should be expected because of greater specificity of the methods?
Synermed's total and direct bilirubin are very specific compared with most reference laboratory methods. The chromatic effects on bilirubin have already been discussed.
Synermed's creatinine method is highly specific for creatinine. Most reference laboratory methods measure glutathione, cystine, cysteine, ergothioneine and protein in addition to creatinine. The result of such specificity is that Synermed can detect very low creatinine values.
Synermed's calcium is specific for calcium in the presence of serum magnesium. Some reference laboratory calcium values are affected by increases or decreases in magnesium.
Connect with us
Certifications
 |
 |
 |
 |
||

