Infrared Accuracy
Infrared Accuracy For Today's Underwriting
Gail S. Page
Assistant Vice-President
Corporate Automation/Scientific Policies
Roche Biomedical Laboratories, Inc.
Burlington, North Carolina
Introduction
The routine clinical chemistry profile is a major screening tool for physicians. Using the values obtained on the chemistry profile as one element of the patient assessment, the physician can determine the presence of disease states such as diabetes alcoholism, and coronary artery disease. The physician can also use the data from chemical profile to predict potential problems before the patient symptoms.
Reliance is placed on the accuracy of results obtained from the chemistry profile in making a patient diagnosis; however, recent studies have raised concerns regarding the accuracy of results obtained from specimens that contain interfering substances. In 1987, Glick and Ryder published an extensive study of the effects of serum chromatic substances on the accuracy of chemistry results obtained on a variety of automated analyzers.1 Serum chromatic substances are those substances endogenous to blood that can color the serum. These include hemolysis, lipemia, and bilirubin. Any serum sample that has high levels of hemolysis, lipemia, or bilirubin absorbs strongly in the ultraviolet and visible potion of the spectrum. Since virtualy all current generation diagnostic methods employ visible or ultraviolet detection means, these chromatic substances often interfere with the results of the assay. For example, consider a patient with hyperlipemia and a dangerously low glucose level of 30mg%. A high level of lipids in the serum could give a falsely elevated absorbance that would result in the calculation of a falsely high glucose result. The patient who is actually hypoglicemic but whose test results are reported as a hyperglicemic due to interference from the lipids, could receive inappropriate therapy.
Dr. Glick has estimate that as many as 30% of samples presented to clinical laboratories have levels of chromatic substances that can produce significant errors in laboratory results. Samples collected for insurance testing are drawn at diverse sites and must be shipped to central laboratories for processing. Rigorous control of fasting states is necessary to minimize lipemia. The trauma of shipping samples can damage red cells, producing hemolysis. Thus, the problem associated with collection and handling predispose insurance samples to the presence of chromatic substances.
The study by Glick and Ryder revealed that all analyzers used to produce clinical chemistry diagnostic results suffer from errors due to interferences from serum chromatic substances. Dr. Glick concludes that three are wide differences in accuracy between analyzer. He writes, "Analyzers that emerge high in the rankings are those that incorporate a successful blending of sample blanking, choice of wavelength(s), timing intervals, sample-to-reagent ratio, reagent formulation and post analysis data manipulation."1(P1457)
Instrument manufacturers have responded to the Glick study by providing improved instrumentation with sample blanking and bichromatic wavelength selection to minimize the interference from serum chromatic substances. Such automation is often sold as a system consisting of the instrument plus the reagents and methods supplied for use on the instrument. As a result, there is a need for studies to evaluate (a) the efficacy of the improvements made by instrument manufactures, and (b) the effects of new reagent technology as applied to these new analyzer. Roche Biomedical Laboratories, based in Burlington, NC, has recently completed a study of two of the new generation analyzer, using reagents and methods provided by the instrument manufacturer. Additionally, a study of the effects on accuracy of a new reagent technology as applied to the most widely used of these analyzers was undertaken. The following data are significant in evaluating the results of the routine diagnostic tests used for insurance purposes.
Materials and Methods
Roche Biomedical evaluated the AU5000 analyzer from Olympus Corporation and the DAX analyzer from Technicon. Both systems have the capability to do dynamic serum blanking and bichromatic interference correction, wich the Glick study cites as important in reducing interferences. Second, Roche evaluated the performance of the Olympus AU5000 with reagents manufactured by Merck. These data were then compared with the results obtained using the AU5000 with reagents manufactured by Synermed, Inc., of Montreal, Canada. Synermed has used a variety of newly synthesized chromogens to enhance routine clinical chemistry tests by producing dramatic improvements in the signal to noise relationship in the Synermed chemistry methods. The basis of signal-to-noise enhancement in these new methods is simple.
It has long been known that hemoglobin and bilirubin have virtually no absorbance in the near-infrared region of the spectrum. The absorbance of chromatic substances progressively diminishes as the wavelength is varied from the ultraviolet to the Infrared. Infrared detection, therefore, greatly diminishes the "noise" due to the absorbance of these substances. In general, the absorbance of bilirubin and hemoglobin is eliminated and the effects of lipemia are reduced to a fraction of its contribution in visible or ultraviolet methods.
The signal portion of the signal-to-noise ratio has been enhanced by the use of chromogens that optimize sensitivity.
This increased sensitivity also allows more optimal sample-to-reagent ratios, thereby diminishing chemical interferences due to matrix effects.
The diminished absorbance due to chromatic substances (noise) and the increased sensitivity (signal) combine to multiply the signal-to-noise ratio. That is, if chromatic absorbance is one fourth as great and the sensitivity is doubled, the signal-to-noise enhancement is 800%.
Beginning from a simple theory, Synermed developed chromogens producing near-infrared absorbing chromophores with optimal sensitivity. The chromogens were configured to use infrared wavelengths available on automated systems in use in laboratories today.
The purpose of the Roche Biomedical study was to explore the comparative efficacy of the two instrument systems and three reagent systems in producing clinically useful results in sera containing both common chromatic substances and commonly encountered drugs.
The evaluation consisted of two Phases: (a) performances studies of sera with the addition of known amounts of chromatic substances, and (b) performance studies of sera with known amounts of commonly used drugs.
Preparation of Interferents
Phase I. Chromatic Substances
20 mg% bilirubin prepared in phosphate buffer
30 mg% hemoglobin prepared by multiple saline washes of packed fresh red blood cells, followed by deionized water lysis and then by centrifugation with toluene to remove cell wall components
1000 mg% tryglyceride solution prepared by aqueous dilution of Intralipid (KabiVitrum, Inc., Alameda, CA)
PhaseI I. Common Drugs
2.0 mg% ascorbic acid
2.0 mg% salicylate. This level is equivalent amount expected from the ingestion of one aspirin tablet. The therapeutic level in arthritic condition is 15 mg% to 30 mg%.
1.0 mg% Dextran prepared by aqueous dilution of commercial 10% Dextran plasma extender. This level equates to the level of treatment after a trauma.
Instrumentation
Olympus AU5000-Olympus Corporation, Lake Success, NY
Technicon DAX- Technicon Coporation, Tarrytown, NY
Reagents
Olympus-Manufactured for Olympus Corporation by Merck DAX-Technicon reagents were obtained from Technicon Corporation
Synermed-Synermed reagents were obtained from Synermed, Inc.
Results
Phase I Study
Specimens were assayed according to the instrument application notes provided by Olympus for the Merck reagents, by Synermed for the Synermed reagents and by Technicon for the DAX reagents. The data which follow are based in within-run analyses to eliminate run to run instrument variation. The bilirubin assay was not evaluated for bilirubin interference for obvious reasons and Triglyceride was not assayed for lipid interference due to the presence of lipase which breaks down triglycerides for the assay and therefore clears specimen. The Phosphorus assay was not evaluated for bilirubin interference due to the method of preparation of the Bilirubin solution (prepared in a phosphate buffer). Hemoglobin interference in the LDH assay was not evaluated due to the presence of erythrocyte LDH from the method of preparation.
The data are expressed as percentage error caused by interference from the chromatic substance expressed as a percent of the normal range.
Combined Report of Percent Error Due to Chronic Interference
All Analysis Methods
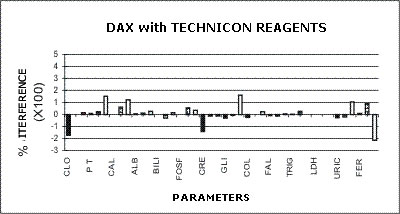
DAX - TECHNICON REAGENTS
| Chlor | PT | CA | Alb | Bili | Phos | Crea | Gluc | Chol | xxx | Trig | LDH | UA | Iron | |
| -175% | 8% | 0% | -143% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | Bili | ||
| -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% |
| -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% | -175% |
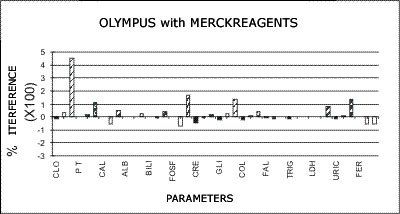
OLYMPUS - MERCK REAGENTS
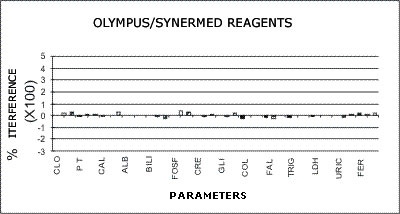
OLYMPUS - SYNERMED REAGENTS
The following graphs represent the data produced during the study with emphasis placed on relative accuracy. The data are based percentage of error created by the interference from bilirubin, hemolysis, or lipids as a ratio of that error to the normal range span when assaying a normal serum. This is almost directly a measure of clinical relevancy of an assay error. For example, the normal range span of triglycerides is 119 mg% and the normal range span of chloride is only 8 mEq/dL.
 |
 |
 |
 |
An arbitrary ±20% of the normal range span has been plotted on the graph as an "acceptable" error level. Some consideration should be given to the fact that this is a "worst case" error level, in that often specimens with such high levels of chromatic interference may be treated separately in the laboratory to avoid these errors. Particular attention should be given to those results that far exceed the ±20% since this indicates that much lower levels of these chromatic interferences would produce clinically invalid results. Such levels of chromatic substances tested are commonplace and virtually unavoidable in the routine laboratory.
Phase II Study
Chromatic interferences can be observed with the laboratorian's eye; however, commonly used, over-the-counter medications cannot be visually observed in the serum. It has been well documented that drugs can cause significant errors in clinical chemistry results (see Young et al). 3 Due to the potential for seriously misleading diagnostic and prognostic information caused by errors from certain medications, the Phase II Study was undertaken. This included a comparative study between the AU5000 with Merck reagents and the AU5000 with Synermed reagents. No drug studies were performed on the DAX system; however, inspection of the methodology employed leads to the conclusion that interferences would be similar to the AU5000 system with Merck reagents. In particular salicylate interference of the Merck reagents (see below).
Merck Reagent Systems as Applied to the AU5000
Chloride
A positive error greater than the normal range is encountered with salicylate levels that are equivalent to ingestions of only one aspirin tablet. Therapeutic levels used in the treatment of arthritis may cause coupling of the result. The presence of such error would create an impossible situation in any attempt to restore electrolyte balance to the patient with salicylate toxicity. The chloride test is one example of a test the results of which may be used to control electrolyte therapy. The New England Journal of Medicine has presented facts recommending that all patients beyond a certain age take low doses of salicylate; hence, it is possible that analysis on patients past 40 may be questionable.
Total Protein
Dextran (plasma expander) was found to give 100% errors in the concentration tested. In addition, since the problem is one of massive precipitation, the total protein module on any instrument is virtually shut down when these specimens are encountered.
Uric Acid
Variation in ascorbic acid concentration was found to produce greater variation in the uric acid test than normal uric acid variation. The high normal ascorbic acid concentration tested will reduce a 5.0 mg% uric acid to 2.0 mg%. Higher levels have an even greater effect. There is a more insidious problem: Because ascorbic acid is unstable, a test performed on fresh serum will give one answer and a repeat at a later time a much different answer, leading the operator to assume that the test lacks reproducibility.
Cholesterol
Reduction of cholesterol values of up to 20% were encountered with normal levels of ascorbic acid. The high levels which may be encountered should a patient ingest the megadoses of Vitamin C - available over-the-counter and often recommended in professional and unprofessional nutritional literature as beneficial in avoiding the common cold - would cause dramatic decrease in apparent cholesterol level. Repeat testing of the same patient at differing times or with differing delays before analysis would produce errors of up to 50% of the original value depending upon how elevated the original value was.
Other Tests
All other AU-5000-Present Methodology tests were affected to a lesser extent than those above by variation in ascorbic acid levels or the potential implication of those effects were less.
Synermed Reagent Systems as Applied to the AU5000
No significant effects were noted from any of the drugs tested. There was no effect on the chloride result from the addition of salicylate. Only a 12% bias was noted from the high level of dextran used for interference testing in the total protein method. A slight effect was noted from ascorbic acid interference in the peroxidase methods.
Glick Study Comparison
The paper published by Glick teaches a method for ranking instruments for their freedom from chromatic interferences. A perfect score is 1.00 and the higher the score, the more interference from chromatic substances. In his seminar, Dr. Glick draws an "acceptable-unacceptable" line about 1.6. Systems with a score of less than 1.6 are acceptable an those with scores higher than 1.6 are unacceptable.
The estimated ranking of the three studied instrument-reagent combinations are:
AU5000-Synermed reagents 1.14
DAX-Technicon reagents 1.60
AU5000-Merck reagents 1.70
In the figure below, the above rankings have been superimposed on a figure reproduced from the Glick study of other instrument tested.1 Note that while both the AU5000 with Merck reagents and the DAX with Technicon reagents are similar to other widely used instruments, the AU5000 with Synermed reagents achieves unprecedented freedom from chromatic interferences.
Conclusions
Laboratories should optimize the reagents used on the new generation automation and should not rely on a system that includes reagents provided by manufacturer without testing interferences. The interfering substances evaluated should include chromatic interferences as well commonly used over-the-counter medication. Roche Biomedical Laboratories has concluded that the new Synermed near-infrared reagent technology represents a significant advancement in the state-of-the art for reagents that are used in routine clinical chemistry today. New generation automation, combined with the use of infrared-enhanced interference correction with high signal-to-noise ratios can enable a new standard for accuracy. The ability to virtually eliminate interference from turbidity, homolysis, and bilirubin as well as certain common over-the-counter drugs allows a more accurate assessment of the patient's condition and prognosis. Such improvements are vital in overcoming the problems associated with the accurate analysis of specimens contaminated with interfering substances that are presented for analysis to today's laboratory.
Connect with us
Certifications
 |
 |
 |
 |
||

