About Us
 Synermed International Inc. is a development based diagnostic biochemistry company, established in 1989. Since that time, the Indianapolis, Indiana headquartered Company has invested millions of dollars in research toward the development of a new generation of diagnostic reagents and instruments.
Synermed International Inc. is a development based diagnostic biochemistry company, established in 1989. Since that time, the Indianapolis, Indiana headquartered Company has invested millions of dollars in research toward the development of a new generation of diagnostic reagents and instruments.
Infrared Laboratory Systems, LLC (ILS) is engaged in the research, development, manufacture, sales and service of in vitro diagnostic reagents and automation for use in clinical chemistry and cytology worldwide. The ILS quality system is a continuation of the Synermed International Inc. established in 1989. Quality System and all ILS personnel, equipment and facilities were conducting these activites for Synermed International prior to August 1, 2011. Synermed filed and received 510(k) approval to market the first of its products in the United States in 1990 and had continued to add to the product line with new chemistry methodologies. As of August 2005, Synermed has filed submissions with the FDA and has received 510(k) clearance to market over 40 in vitro diagnostic chemistry methodologies and instruments. Those 510(k) clearances and all Synermed quality records are now the property of Infrared Laboratory Systems. ILS was 88% owned by Synermed International Inc. Shareholders at the time of the quality system transfer.
ILS has U.S. and certain foreign rights to the Synermed® trade mark and manufactures Synermed® brand products.
The chemistry products manufactured by ILS are unique in the marketplace in that only a few other companies manufactures a line of liquid-stable, ready-to-use reagents. The ILS products also include reagents that utilize chromophores that can be read spectrophotometrically in the near infrared. The advantage of reading in the near-infrared is that there is less interference from serum chromatic substances such as hemoglobin, lipemia and bilirubin. In addition, ILS products have been tested and proven to provide accurate results in serum from patients containing hemoglobin-based blood substitutes.
ILS’s Synermed® brand instrument products are being marketed in the U.S.. The instruments optimally utilize the ILS Synermed® brand near-infrared reagents and provide customers with more accurate results in a cost-effective manner.
ILS is also the contract manufacturer of CellSolutions cytology products.
ILS complies with all FDA requirements as established by law and by the Quality System Regulations of 1996: 21 CFR 820. In addition, Synermed operates under the quality system as defined by ISO13485:2003 EC IVDMDD and CMDR to be able to meet the needs of its customers throughout the world. A systematic, information based Risk-Management program has been implemented in accordance with ISO 14971:2007.
Synermed® has developed two reagent technologies which are unique in the diagnostic biochemistry world.
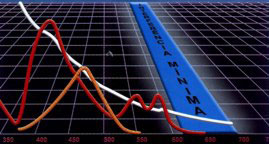 NEAR INFRARED DETECTION
NEAR INFRARED DETECTION
Hemolysis/Icterus/Turbidity (HIT) Graph
Synermed's N-IR Detection enables widely used automation to avoid serious inaccuracies due to lipemia, hemolysis, and icterus.
 LIQUID-STABLE READY-T0-USE REAGENTS
LIQUID-STABLE READY-T0-USE REAGENTS
Most Synermed® reagents are supplied in liquid form and are ready-to-use as supplied, conferring considerable economic advantages and on-board stability.
REAGENT PRODUCTS
Synermed leads the world in technology for stabilizing enzymes and coenzymes and in the synthesis of chromogens producing chromophores absorbing at the 660 and 700 nm wavelengths available on most modern biochemistry automation.
SGS ISO Certification
SYNERMED IS AN FDA GMP AND ISO 13485 CERTIFIED DEVELOPER AND MANUFACTURER
ISO 13485ISO 13485 UKAS
Synermed's reagents are used world wide.
INSTRUMENTATION
Synermed is completing Design Control and regulatory preparations for its new generation Bench-Top analyzer, the Synermed IR500-II, following which Synermed plans to complete its high capacity system. Synermed is optimally applying its interference-free infrared chemistry methods in combination with its ready-to-use liquid-stable reagents to economical and highly efficient systems of automation for laboratories of all sizes.
Synermed expects to announce availability of the first of these systems, the IR500-II ISE, following completion of FDA studies.
Connect with us
Certifications
 |
 |
 |
 |
||

